FROM INVENTION TO SUCCESS
About Turon Medtech
For four decades, Turon Medtech has contributed to the development of stereotactic breast biopsy. Since introducing Cytoguide, the world’s first automated positioning tool for breast biopsy, the company has focused on advancing solutions for precise and efficient procedures.
With a dedicated focus on stereotactic breast biopsy, Cytoguide has been integrated into more than 15 mammography models and adopted by several major manufacturers. These integrations reflect an approach built on engineering expertise, clinical understanding and close collaboration with users and partners.
Turon Medtech is characterized by long-term thinking, experience, and flexibility, combined with a strong commitment to customer-driven and efficient development. High service standards, fast delivery, and close cooperation with both customers and suppliers are central to how we work.
Our in-house capabilities in R&D, manufacturing, service, and regulatory compliance support the entire product lifecycle. Operations follow a quality management system aligned with ISO 13485 and MDR requirements, emphasizing reliable delivery, responsive support, and practical adaptation to customer needs.
Through continuous development, Turon Medtech aims to help shape safer, more efficient, and innovative healthcare. Current concept work focuses on improving safety and workflow across the entire pathway—from screening to biopsy—while exploring advanced technologies. These initiatives form part of a long-term vision to deliver solutions that support progress in breast health diagnostics.
From Invention to Innovation
A Timeline of Innovation: Mammography, Biopsy, and Turon Medtech
The story of mammography spans more than a century, beginning in 1913 with the world’s first mammographic images. Since then, the field has advanced through major milestones, from the introduction of screening programs and stereotactic biopsy to the emergence of digital imaging, tomosynthesis, and AI-driven tools.
Turon Medtech became part of this evolving landscape in the mid-1980s, contributing solutions that advanced precision and efficiency in breast biopsy. From the pioneering introduction of Cytoguide, through its continuous refinement and adaptation, to today’s ongoing concept work, the company’s journey reflects an enduring commitment to clinical needs and technological progress.
The timeline below brings together key moments in mammography and stereotactic biopsy, alongside steps that illustrate Turon Medtech’s role in this shared history. Explore how decades of innovation have shaped today’s standards—and continue to influence what comes next.
Patent for Cytosafe

Responding to market feedback, Turon Medtech is continuing to develop Cytosafe; a solution designed to simplify biopsy procedures and enhance safety. Cytosafe aims to reduce contamination risk and ensure correct needle sizing. In 2025, the product was patented, marking a significant milestone in an ongoing development process that reflects Turon’s commitment to innovation and future-ready healthcare solutions.
Turon Opens R&D Office in Halmstad

To accelerate innovation, Turon Medtech established a dedicated research and development center in Halmstad in 2023. The facility features advanced in-house 3D printing capabilities using SLA (Stereolithography) and FDM (Fused deposition modeling) technologies, enabling rapid prototyping and small-scale production.
Concept Development for the Future of Biopsy
In 2022, Turon Medtech began a strategic program to redefine stereotactic- and tomobiopsy workflows. The focus is on improving safety and efficiency throughout the entire pathway, from screening to biopsy, while incorporating advanced technologies such as AI. These developments are a central part of Turon’s long-term vision to deliver innovative and dependable healthcare solutions.
AI Enters Clinical Screening

After years of research, artificial intelligence began to be implemented in clinical mammography workflows around 2021. Based on deep learning, the technology provides risk scores, which estimate the likelihood of cancer for each case, and lesion markings, which highlight suspicious areas on the image to guide radiologists. Large scale studies show that AI can increase cancer detection by 20 – 30 percent while reducing unnecessary recalls, making the process both safer and more efficient. This is regarded as the next major step in the evolution of breast cancer screening.
Upgraded Hand Control and Electronics

To meet market demands, Cytoguide’s hand control was redesigned in 2020 with tactile feedback for precise manual positioning. In 2021, a major electronics upgrade followed, maintaining performance and preparing the system for future requirements.
Enhancing Flexibility in Biopsy Solutions
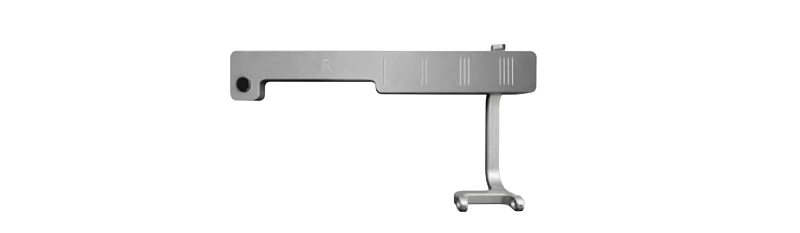
In 2018, Turon Medtech developed additional needle holders to fully support both lateral and vertical vacuum-assisted biopsies, optimized for all major VAB needle types. In 2019, a lateral holder for fine- and core-needle biopsies was launched, broadening clinical flexibility and application.
Cytoguide Adapts to Tomosynthesis Biopsy
By 2017, Cytoguide became fully compatible with tomosynthesis guided biopsies, 3D imaging instead of traditional 2D projections. This advancement reduced the risk of lesions being hidden by overlapping tissue and improved targeting accuracy, especially for small or subtle findings. At the same time, Turon introduced the Quick Mount Adapter system. The system aimed to simplify the handling of needle holders while also being transparent during tomosynthesis guided biopsies with both fine and core needles, streamlining procedures and increasing precision. Turon Medtech also expanded its internal capabilities with 3D printers for rapid prototyping, SLA (Stereolithography) and FDM (Fused deposition modeling) printing.
Acquisition by PS Provider Group

In 2016, Turon Medtech joined the PS Provider Group, marking a new chapter of strategic development and expanded resources. At this time, Jonas Lindquist gradually transitioned out of his operational role, ensuring a smooth handover. He continues at serve as a valued mentor, supporting Turon Medtech’s commitment to excellence and innovation.
Introduction of the Third-Generation Cytoguide
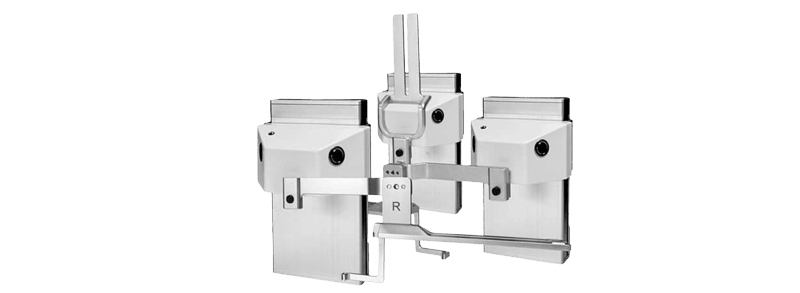
In 2015, Turon Medtech introduced the third-generation Cytoguide, with a clear focus on user needs and biopsy workflow. The upgrade added new features to enhance safety, streamline procedures, and simplify operations. These improvements made the system more intuitive and efficient, reinforcing its global market position.
Tomosynthesis Brings Mammography into 3D
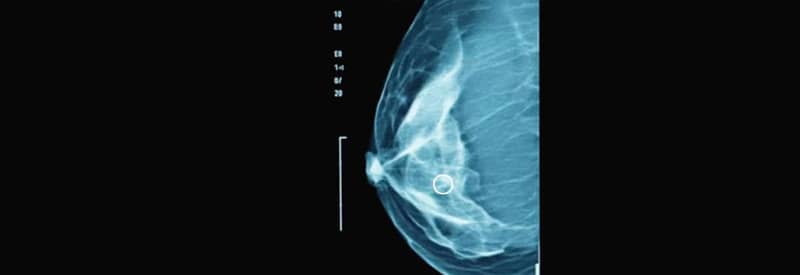
Digital breast tomosynthesis (DBT), also known as 3D mammography, emerged as an evolution of traditional 2D imaging. This technology creates thin sectional images of the breast, making it easier to detect small or hidden lesions, especially in dense breast tissue. The FDA approved the first commercial system in 2011, and major studies throughout the decade showed that DBT could increase cancer detection by 30 to 40 percent while simultaneously lowering the amount of false-positive results compared to 2D imaging. During the late 2000s and early 2010s, vacuum assisted.
Digital Transformation

The new millennium introduced digital mammography, approved by the FDA in 2000. Digital technology streamlined workflows, eliminated film processing, and enabled advanced image manipulation along with PACS (Picture Archiving and Communication Systems) archiving, used to store and share medical images electronically. The second generation Cytoguide evolved to integrate seamlessly with digital systems, ensuring continued leadership in precision biopsy. Turon Medtech strengthened global partnerships, collaborating with major players such as Siemens and further expanding its reach across the globe.
Turon Medtech Becomes Independent

In 2000, Turon Medtech AB was established as an independent entity, breaking away from Turon AB under the leadership of Jonas Lindquist. With visionary drive and entrepreneurial spirit, Jonas transformed Turon Medtech into a thriving company, laying the foundation for its future growth and innovation in stereotactic breast biopsy solutions.
Expansion and Technological Shifts
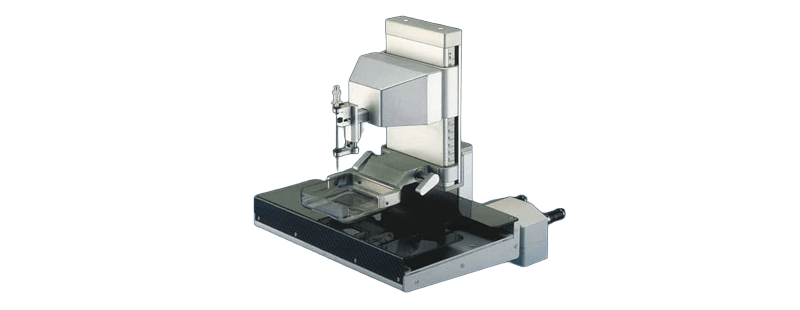
Throughout the 1990s, the first-generation analog Cytoguide system gained worldwide adoption, integrated into systems from leading manufacturers such as Planmed and Toshiba. Meanwhile, biopsy techniques advanced from fine needle aspiration to core biopsy, and later vacuum assisted biopsy (VAB). VAB uses a vacuum powered device to collect larger tissue samples. This method also enables percutaneous removal, meaning removal through the skin without open surgery, of suspicious calcifications and minimizes surgical interventions. The first vacuum assisted biopsy device received FDA approval in 1995. Its use expanded quickly in the United States, while spread in other regions was more gradual.
The Birth of Turon Medtech
Amid Sweden’s national screening rollout, Inventor Björn Ericson together with Chief Physician Bjurstrand at Sahlgrenska University Hospital initiated development of Cytoguide—the world’s first automated positioning system for breast biopsy. The first prototype appeared in 1986, followed by global commercialization of the first generation in partnership with Philips in 1987. Cytoguide became a milestone in stereotactic biopsy, setting new standards for precision and efficiency.
Stereotactic Breast Biopsy – A Swedish Breakthrough
Stereotactic breast biopsy, which uses three dimensional coordinates to sample non-palpable lesions, meaning small abnormalities that cannot be felt during a physical exam, was pioneered in Sweden alongside early screening programs. Karolinska Institutet in Stockholm led the world in clinical application, thanks to innovators Sven K. Franzén and Josef Zajicek, both renowned pathologists who advanced breast cancer diagnostics. The first stereotactic biopsy table was built in the early 1980s, reducing unnecessary surgery for benign findings, which are noncancerous tissue changes. The technique quickly spread internationally, with U.S. adoption in 1986 and 1987 directly influenced by Swedish expertise.
The Screening Era and Sweden’s Pioneering Role

The 1970s marked a shift from diagnostic mammography to preventive screening. The HIP study in New York, a large clinical trial, showed that regular mammography could reduce breast cancer mortality by about 30 percent. Sweden emerged as a global leader: Bengt Lundgren’s low dose technique in Gävleborg minimized radiation exposure without compromising image quality, and László Tabár’s landmark Two County Trial (1977–1985), a randomized study involving thousands of women in two Swedish counties, demonstrated that organized screening programs significantly reduce breast cancer deaths. Sweden introduced nationwide breast cancer screening in 1986, supported by studies in Malmö, Stockholm, and Gothenburg, cementing its position at the forefront of screening innovation.
The Rise of Modern Mammography
During the 1950s and 1960s, mammography evolved from basic X-ray images to dedicated high resolution breast imaging systems. Innovations by Robert Egan improved film techniques, enabling detection of small nonpalpable tumors. Advances such as xeromammography, a dry imaging process that offered clearer soft tissue contrast, and molybdenum based X-ray tubes, which provided optimal energy for breast tissue, dramatically enhanced image quality and established the basis for today’s reliable breast cancer diagnostics.
The Origins of Mammography
In 1913, German physician Albert Salomon captured the world’s first mammographic images by X-raying surgically removed breasts to study cancerous tissue. His pioneering work revealed how different breast tumors appeared on X-ray and how they spread to lymph nodes, laying the foundation for modern breast imaging.


